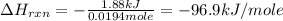Chemistry, 16.10.2019 03:30 kealinwiley
You place 36.5 ml of 0.266 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 56.6 ml of 0.648 m hcl, also at 25.00°c. after stirring, the final temperature is 29.83°c. {assume that the total volume is the sum of the individual volumes and that the final solution has the same density (1.00 g/ml) and specific heat capacity (4.184 j/g°c) as water}. calculate the change in enthalpy, δh, of the reaction (in kj/mol) of water formed. enter the appropriate sign (+/

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1


Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
You place 36.5 ml of 0.266 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 56.6 ml of 0.648...
Questions




Mathematics, 05.10.2019 12:30


History, 05.10.2019 12:30






Computers and Technology, 05.10.2019 12:30

Mathematics, 05.10.2019 12:30

Mathematics, 05.10.2019 12:30

Social Studies, 05.10.2019 12:30


Mathematics, 05.10.2019 12:30


Mathematics, 05.10.2019 12:30

History, 05.10.2019 12:30

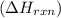 is, -96.9 kJ/mole
is, -96.9 kJ/mole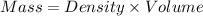
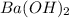
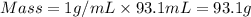
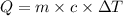
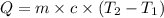
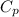 = specific heat capacity of water =
= specific heat capacity of water = 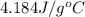
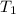 = initial temperature =
= initial temperature = 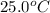
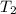 = final temperature =
= final temperature = 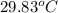
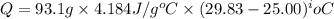
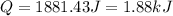 (1 kJ = 1000 J)
(1 kJ = 1000 J)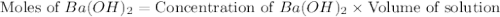
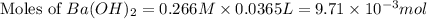
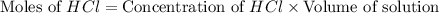
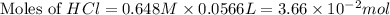
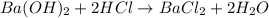
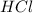
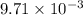 moles of
moles of 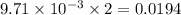 moles of
moles of 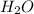
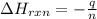
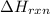 = enthalpy of reaction = ?
= enthalpy of reaction = ?