Chemistry, 16.10.2019 05:00 aesmithswhs
Which solution will have the highest ph? the ka of hclo is 3.8 × 10−8.(a) a solution of 100 ml of water with 20 ml of 0.1 m naoh added.(b) a 100 ml buffer solution of 0.1 m hclo, 0.1 mclo− with 20 ml of 0.1 m hcl solution added.(c) a buffer solution of 0.1 m hclo, 0.1 m clo−.(d) a 100 ml buffer solution of 0.1 m hclo, 0.1 m clo− with 20 ml of 0.1 m naoh solution added.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
Which solution will have the highest ph? the ka of hclo is 3.8 × 10−8.(a) a solution of 100 ml of w...
Questions

Mathematics, 23.10.2020 18:20


Mathematics, 23.10.2020 18:20

Chemistry, 23.10.2020 18:20


Mathematics, 23.10.2020 18:20

History, 23.10.2020 18:20

Computers and Technology, 23.10.2020 18:20

History, 23.10.2020 18:20



Mathematics, 23.10.2020 18:20

Mathematics, 23.10.2020 18:20

English, 23.10.2020 18:20




Mathematics, 23.10.2020 18:20


Social Studies, 23.10.2020 18:20

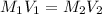
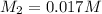
![[OH^-] = 0.017 M](/tpl/images/0324/1344/85aab.png)
![pH = pK_a + log\frac{[salt]}{[acid]}](/tpl/images/0324/1344/d1225.png)