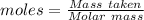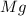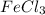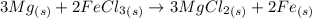Chemistry, 16.10.2019 06:00 jessica28757
Magnesium reacts with iron(iii) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron. 3mg(s) + 2fecl3(s) → 3mgcl2(s) + 2fe(s) a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. what mass of magnesium chloride = 95.21 g/mol) is formed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Magnesium reacts with iron(iii) chloride to form magnesium chloride (which can be used in fireproofi...
Questions


History, 30.09.2021 03:10

Mathematics, 30.09.2021 03:10

Chemistry, 30.09.2021 03:10


Mathematics, 30.09.2021 03:10

Biology, 30.09.2021 03:10


Mathematics, 30.09.2021 03:10

English, 30.09.2021 03:10

Mathematics, 30.09.2021 03:10

Mathematics, 30.09.2021 03:10




Mathematics, 30.09.2021 03:10

Mathematics, 30.09.2021 03:10


English, 30.09.2021 03:10