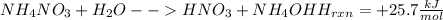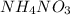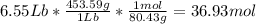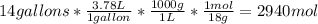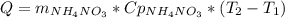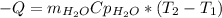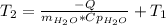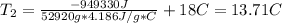Acertain commercial process relies on the addition of nh4no3 to a solution of their proprietary compound. the junior apprentice chemist develops a plan to add 6.55 pounds of nh4no3 to 14.0 gallons of water. the water in the factory is typically at an initial temperature of 18.0 c, and the temperature of the water cannot drop below 15 c, or the reaction will be too slow. will the above plan work? use the value of delta hrxn = +25.7 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which phrase best describes the rock's texture? 1.jagged grains 2.coarse grains 3.rounded grains 4.non-banded grains
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3
You know the right answer?
Acertain commercial process relies on the addition of nh4no3 to a solution of their proprietary comp...
Questions

Social Studies, 21.04.2020 17:00



Mathematics, 21.04.2020 17:00


English, 21.04.2020 17:00








Health, 21.04.2020 17:00

Mathematics, 21.04.2020 17:00



Social Studies, 21.04.2020 17:00

Mathematics, 21.04.2020 17:00

Mathematics, 21.04.2020 17:00