
Chemistry, 16.10.2019 21:20 sdwhitneyhillis
The rate of decay of a chemical involved in a reaction that is second order (bimolecular) in one reactant a is given by: = k [aj? -d[a/dt where k is the reaction rate coefficient. derive an expression for the half-life of a in terms of k and the concentration of a at time t-0 (ao)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1


Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1
You know the right answer?
The rate of decay of a chemical involved in a reaction that is second order (bimolecular) in one rea...
Questions




















Computers and Technology, 13.04.2021 02:10

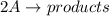
![\textrm{rate}=-\dfrac{\Delta[\textrm A]}{2\Delta t}=k[\textrm A]^2](/tpl/images/0326/2107/66a24.png)
![\dfrac{d[A]}{dt}=-k[A]^2](/tpl/images/0326/2107/6f64e.png)
![\int_{[A_t]}^{[A_0]}\frac{d[A]}{[A]^2}=-\int_{0}^{t}kdt](/tpl/images/0326/2107/7af1b.png)
![\dfrac{1}{[A]} = \dfrac{1}{[A]_0}+kt](/tpl/images/0326/2107/704d4.png)
![[A_t]](/tpl/images/0326/2107/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0326/2107/9a686.png) is the initial concentration
is the initial concentration![[A_t]=\frac{1}{2}\times [A_0]](/tpl/images/0326/2107/09fb2.png)
![t_{1/2}=\dfrac{1}{k[A_o]}](/tpl/images/0326/2107/4b6c5.png)


