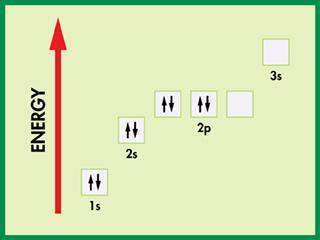
the orbital diagram below is written incorrectly. why?
a. the number of electrons used...

Chemistry, 17.10.2019 00:00 kasonlowery
the orbital diagram below is written incorrectly. why?
a. the number of electrons used is incorrect.
b. the orbitals are not in the correct sequence.
c. the orbital diagram violates hund's rule.
d. the electrons are all paired.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Questions

History, 09.07.2019 09:30



Mathematics, 09.07.2019 09:30


Mathematics, 09.07.2019 09:30

History, 09.07.2019 09:30





English, 09.07.2019 09:30

English, 09.07.2019 09:30



History, 09.07.2019 09:30

Mathematics, 09.07.2019 09:30



Mathematics, 09.07.2019 09:30



