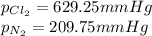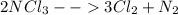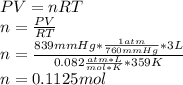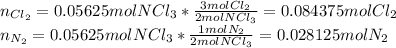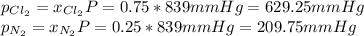Be sure to answer all parts. liquid nitrogen trichloride is heated in a 3.00−l closed reaction vessel until it decomposes completely to gaseous elements. the resulting mixture exerts a pressure of 839 mmhg at 86°c. what is the partial pressure of each gas in the container?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
Be sure to answer all parts. liquid nitrogen trichloride is heated in a 3.00−l closed reaction vesse...
Questions


English, 30.10.2020 02:40


Mathematics, 30.10.2020 02:40

History, 30.10.2020 02:40


English, 30.10.2020 02:40



Business, 30.10.2020 02:40



English, 30.10.2020 02:40

Mathematics, 30.10.2020 02:40


Mathematics, 30.10.2020 02:40



Mathematics, 30.10.2020 02:40