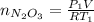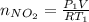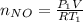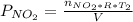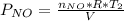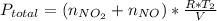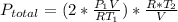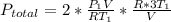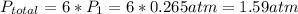Acontainer of n2o3(g) has a pressure of 0.265 atm. when the absolute temperature of the n2o3(g) is tripled, the gas completely decomposes, producing no2(g) and no(g). calculate the final pressure of the gas mixture, assuming that the container volume does not change.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Acontainer of n2o3(g) has a pressure of 0.265 atm. when the absolute temperature of the n2o3(g) is t...
Questions

Mathematics, 10.05.2021 15:10

Mathematics, 10.05.2021 15:10

Mathematics, 10.05.2021 15:10

Mathematics, 10.05.2021 15:10

Chemistry, 10.05.2021 15:10

English, 10.05.2021 15:10


Mathematics, 10.05.2021 15:10


World Languages, 10.05.2021 15:10

Mathematics, 10.05.2021 15:10


Mathematics, 10.05.2021 15:10


Mathematics, 10.05.2021 15:10

History, 10.05.2021 15:10


Chemistry, 10.05.2021 15:10

Health, 10.05.2021 15:10

Geography, 10.05.2021 15:10

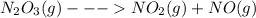
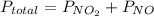
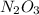 because it decomposed completely.
because it decomposed completely.