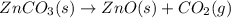The decomposition of znco3(s) into zno(s) and co2(g) at constant pressure requires the addition of 71.5 kj of heat per mole of znco3. you may want to reference (pages 177 - 178) section 5.4 while completing this problem. part apart complete write a balanced equation for the reaction. express your answer as a chemical equation. identify all of the phases in your answer. znco3(s)→zno(s)+co2(g) previous answers correct the reactant and products of the decomposition reaction are given in the introduction. znco3(s) is broken down into zno(s) and co2(g). part b what is the enthalpy of the reaction above?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1


Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
The decomposition of znco3(s) into zno(s) and co2(g) at constant pressure requires the addition of 7...
Questions

Mathematics, 09.02.2021 19:00



Mathematics, 09.02.2021 19:00

Mathematics, 09.02.2021 19:00

Mathematics, 09.02.2021 19:00


Mathematics, 09.02.2021 19:00

English, 09.02.2021 19:00

Chemistry, 09.02.2021 19:00

Mathematics, 09.02.2021 19:00

History, 09.02.2021 19:00

Mathematics, 09.02.2021 19:00

Mathematics, 09.02.2021 19:00


Chemistry, 09.02.2021 19:00

Mathematics, 09.02.2021 19:00