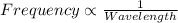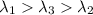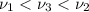One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of 2.12 10-10 m, and another type of electromagnetic radiation has photons with energy equal to 3.97 10-19 j/photon. identify each type of electromagnetic radiation. 107.1 mhz 2.12 10-10 m 3.97 10-19 j/photon fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light rank them in order of increasing photon energy and increasing frequency

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of...
Questions


History, 01.10.2019 02:30

Mathematics, 01.10.2019 02:30

Social Studies, 01.10.2019 02:30

Social Studies, 01.10.2019 02:30

History, 01.10.2019 02:30



Mathematics, 01.10.2019 02:30

History, 01.10.2019 02:30



Mathematics, 01.10.2019 02:30



Mathematics, 01.10.2019 02:30


English, 01.10.2019 02:30

Mathematics, 01.10.2019 02:30

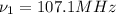
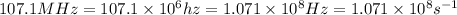
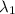
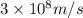
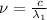
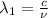
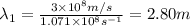
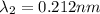 (X-rays)
(X-rays)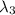
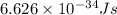
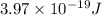
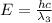
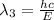
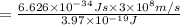
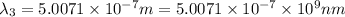
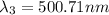 (visible light)
(visible light)