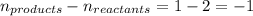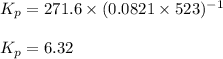Chemistry, 17.10.2019 18:00 pippalotta
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the other phosphorus compounds, coexist in equilibrium through the reaction
pcl3(g) + cl2(g) = pcl5(g)
at 250 ᵒc , an equilibrium mixture in a 25.0 l flask contains 0.105 g pcl5, 0.220 g pcl3 and 2.12 g of cl2. what are the values of
a) kc
b) kp for this reaction at 250 ᵒc ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
The two common chlorides of phosphorus, pcl3, and pcl5, both important for the production of the oth...
Questions

English, 16.07.2020 21:01



Mathematics, 16.07.2020 21:01






Mathematics, 16.07.2020 21:01




Mathematics, 16.07.2020 21:01

Mathematics, 16.07.2020 21:01





Spanish, 16.07.2020 21:01

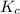 for the given reaction is 271.6
for the given reaction is 271.6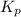 for the reaction is 6.32
for the reaction is 6.32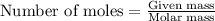 .....(1)
.....(1)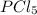 :
: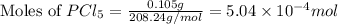
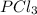 :
: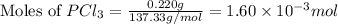
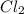 :
: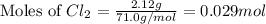

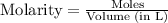
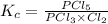
![[PCl_5]=\frac{5.04\times 10^{-4}mol}{25L}](/tpl/images/0328/9204/d1b89.png)
![[PCl_3]=\frac{1.60\times 10^{-3}mol}{25L}](/tpl/images/0328/9204/16be6.png)
![[Cl_2]=\frac{0.029mol}{25L}](/tpl/images/0328/9204/6d85a.png)
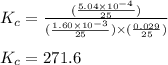
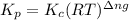
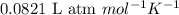
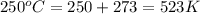
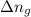 = change in number of moles of gas particles =
= change in number of moles of gas particles =