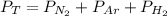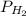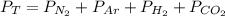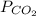Chemistry, 17.10.2019 18:10 shaylasimonds587
Dalton’s law calculationa mixture of h₂, n₂ and ar gases is present in a steel cylinder. the total pressure within the cylinder is 675 mm hg and the partial pressures of n₂ and ar are, respectively, 354 mm hg and 235 mm hg. if co₂ gas is added to the mixture, at constant temperature, until the total pressure reaches 842 mm hg, what is the partial pressure, in mm hg, of the following? a) co₂, 167 b) n₂, 354 c) ar, 235 d) h₂, 8629

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Dalton’s law calculationa mixture of h₂, n₂ and ar gases is present in a steel cylinder. the total...
Questions



Biology, 09.08.2019 19:20
















History, 09.08.2019 19:20

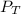 = total presure of the system (675 mm Hg).
= total presure of the system (675 mm Hg).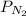 = partial pressure of N₂ (354 mm Hg).
= partial pressure of N₂ (354 mm Hg).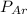 = partial pressure of Ar (235 mm Hg).
= partial pressure of Ar (235 mm Hg).