
The decomposition reaction 2 hi(g) → h2(g) + 12(g), is second order and has a rate constant equal to 1.6 x 10m's at 700 °c. if the initial concentration of hi in the container is 3.4 x 10-m, how many minutes will it take for the concentration to be reduced to 8.0 x 104 m?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
The decomposition reaction 2 hi(g) → h2(g) + 12(g), is second order and has a rate constant equal to...
Questions

Social Studies, 08.06.2021 08:30





Mathematics, 08.06.2021 08:30

Mathematics, 08.06.2021 08:30

Chemistry, 08.06.2021 08:30


Mathematics, 08.06.2021 08:30

History, 08.06.2021 08:30


History, 08.06.2021 08:30

Mathematics, 08.06.2021 08:30

Computers and Technology, 08.06.2021 08:30

Mathematics, 08.06.2021 08:30

Mathematics, 08.06.2021 08:30



Mathematics, 08.06.2021 08:30

![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0329/9761/ccade.png)
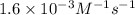
![[A_t]](/tpl/images/0329/9761/5262c.png) = final concentration =
= final concentration = 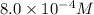
![[A_o]](/tpl/images/0329/9761/dc622.png) = initial concentration =
= initial concentration = 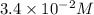
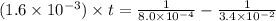
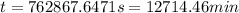 (1 min = 60 s)
(1 min = 60 s)

