
Chemistry, 18.10.2019 01:00 19thomasar
The equilibrium constant kp for the reaction (ch3),cci (g) = (ch3),c=ch, (g) + hcl (g) is 3.45 at 500. k. (5.00 x 10k) calculate the value of kc at 500. k. for the same reaction, calculate the molar concentration of reactants and products at equilibrium if initially 1.00 mol of (ch3),cci was placed in a 5.00 l vessel.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
The equilibrium constant kp for the reaction (ch3),cci (g) = (ch3),c=ch, (g) + hcl (g) is 3.45 at 50...
Questions

Computers and Technology, 01.08.2019 02:00



Business, 01.08.2019 02:00






History, 01.08.2019 02:00


English, 01.08.2019 02:00


Mathematics, 01.08.2019 02:00


English, 01.08.2019 02:00


History, 01.08.2019 02:00

Mathematics, 01.08.2019 02:00

Mathematics, 01.08.2019 02:00

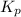 for the reaction is 6.32 and concentrations of
for the reaction is 6.32 and concentrations of 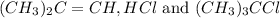 is 0.094 M, 0.094 M and 0.106 M respectively.
is 0.094 M, 0.094 M and 0.106 M respectively.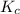 is given by the formula:
is given by the formula: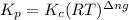
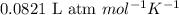
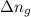 = change in number of moles of gas particles =
= change in number of moles of gas particles = 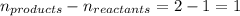
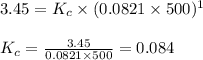
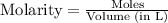
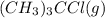 = 1.00 mol
= 1.00 mol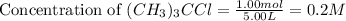
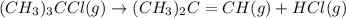
![K_c=\frac{[(CH_3)_2C=CH]\times [HCl]}{[(CH_3)_3CCl]}](/tpl/images/0329/9758/8a400.png)
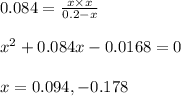
![[(CH_3)_2C=CH]=x=0.094M](/tpl/images/0329/9758/5587a.png)
![[HCl]=x=0.094M](/tpl/images/0329/9758/e3b5a.png)
![[(CH_3)_3CCl]=(0.2-x)=(0.2-0.094)=0.106M](/tpl/images/0329/9758/364df.png)


