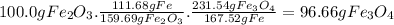calculate the weight of fe3o4 in 100.0g fe2o3.
given: molar mass of fe2o3 =159.69 g/mol...

Chemistry, 18.10.2019 01:00 CameronVand21
calculate the weight of fe3o4 in 100.0g fe2o3.
given: molar mass of fe2o3 =159.69 g/mol molar mass of fe3o4 = 231.54 g/mol hint: you need two conversion factors

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
Questions


Mathematics, 12.07.2021 02:30


Mathematics, 12.07.2021 02:50


Computers and Technology, 12.07.2021 02:50

Medicine, 12.07.2021 02:50

Mathematics, 12.07.2021 02:50

History, 12.07.2021 02:50

Mathematics, 12.07.2021 02:50


Mathematics, 12.07.2021 02:50


English, 12.07.2021 02:50

Mathematics, 12.07.2021 02:50

Spanish, 12.07.2021 02:50



Mathematics, 12.07.2021 02:50