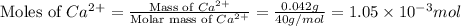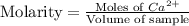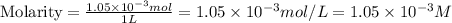Chemistry, 18.10.2019 00:30 hernsl0263
A) a water sample (density=1.00g/ml, s=0.28g/kg) contains ca(2+) at a concentration of 42 mg/kg. calculate the molarity of the ion.

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Asolution is prepared using 0.125 glucose c6h12o6 is enough water to make 250 g of total solution the concentration of the solution, expressed in parts per million is
Answers: 3

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1
You know the right answer?
A) a water sample (density=1.00g/ml, s=0.28g/kg) contains ca(2+) at a concentration of 42 mg/kg. cal...
Questions

Chemistry, 03.03.2021 21:30

Mathematics, 03.03.2021 21:30


English, 03.03.2021 21:30




Mathematics, 03.03.2021 21:30

Mathematics, 03.03.2021 21:30




History, 03.03.2021 21:30


Mathematics, 03.03.2021 21:30

Mathematics, 03.03.2021 21:30


Spanish, 03.03.2021 21:30

Mathematics, 03.03.2021 21:30

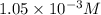
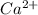 = 42 mg/kg
= 42 mg/kg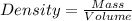
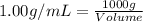
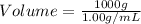
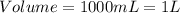 (1 L = 1000 mL)
(1 L = 1000 mL)