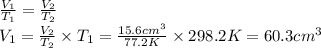Chemistry, 18.10.2019 00:30 lesliealvarado1022
Arubber balloon was filled with helium at 25.0˚c and placed in a beaker of liquid nitrogen at -196.0˚c. the volume of the cold helium was 15.6 cm3. assuming ideal gas behavior and isobaric conditions, what was the volume of the helium at 25.0˚c?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1
You know the right answer?
Arubber balloon was filled with helium at 25.0˚c and placed in a beaker of liquid nitrogen at -196.0...
Questions




English, 09.06.2020 05:57

Mathematics, 09.06.2020 05:57

History, 09.06.2020 05:57






Spanish, 09.06.2020 05:57




Biology, 09.06.2020 05:57


Mathematics, 09.06.2020 05:57


Chemistry, 09.06.2020 05:57