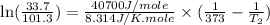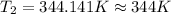Chemistry, 18.10.2019 01:20 dewayne5599
3. at sea level, the atmospheric pressure is 101.3 kpa. atop mount everest, the atmospheric pressure is 33.7 kpa. considering the normal boiling point of water (100 °c) and its heat of vaporization (40.7 kj/mol), at what temperature will water boil atop mount everest? m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3


Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
3. at sea level, the atmospheric pressure is 101.3 kpa. atop mount everest, the atmospheric pressure...
Questions

Mathematics, 21.08.2019 00:50


Chemistry, 21.08.2019 00:50


Mathematics, 21.08.2019 00:50

Mathematics, 21.08.2019 00:50



Physics, 21.08.2019 00:50

Social Studies, 21.08.2019 00:50

English, 21.08.2019 00:50

History, 21.08.2019 00:50

Physics, 21.08.2019 00:50


Physics, 21.08.2019 00:50


Physics, 21.08.2019 00:50

Mathematics, 21.08.2019 00:50

Mathematics, 21.08.2019 00:50

Mathematics, 21.08.2019 00:50

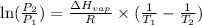
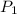 = atmospheric pressure at at sea level = 101.3 kPa
= atmospheric pressure at at sea level = 101.3 kPa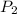 = atmospheric pressure at top mount everest = 33.7 kPa
= atmospheric pressure at top mount everest = 33.7 kPa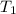 = normal boiling point of water =
= normal boiling point of water = 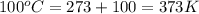
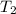 = temperature at top mount everest = ?
= temperature at top mount everest = ?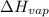 = heat of vaporization = 40.7 kJ/mole = 40700 J/mole
= heat of vaporization = 40.7 kJ/mole = 40700 J/mole