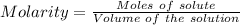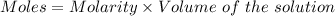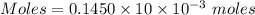Chemistry, 18.10.2019 03:30 tdyson3p6xvtu
2. suppose that 21.37 ml of naoh is needed to titrate 10.00 ml of 0.1450 m h2so4 solution.
(a) write a balanced reaction equation for the acid-base neutralization.
(b) calculate the molarity of naoh based on the results of this titration.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
2. suppose that 21.37 ml of naoh is needed to titrate 10.00 ml of 0.1450 m h2so4 solution.
(a)...
(a)...
Questions



English, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31


English, 28.01.2020 07:31

Mathematics, 28.01.2020 07:31

History, 28.01.2020 07:31


Biology, 28.01.2020 07:31

Biology, 28.01.2020 07:31




Mathematics, 28.01.2020 07:31

Biology, 28.01.2020 07:31

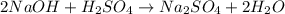
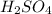 can be calculated as:
can be calculated as: