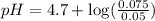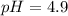Chemistry, 18.10.2019 03:30 zoedaejohnson
Question 2 calculate the ph of a solution that has an acetic acid concentration of 0.05 m and a sodium acetate concentration of 0.075 m. (a) by making use of the henderson - hasselbach equation. (b) without making any assumptions. (4) (6) 1101

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Question 2 calculate the ph of a solution that has an acetic acid concentration of 0.05 m and a sodi...
Questions

Mathematics, 11.02.2021 17:30







History, 11.02.2021 17:30

Arts, 11.02.2021 17:30

Biology, 11.02.2021 17:30

Mathematics, 11.02.2021 17:30





History, 11.02.2021 17:30




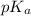 .
.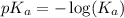
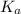 in this expression, we get:
in this expression, we get: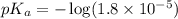
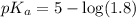
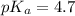
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0330/1774/e961a.png)
![pH=pK_a+\log \frac{[CH_3COONa]}{[CH_3COOH]}](/tpl/images/0330/1774/023d4.png)