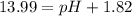Chemistry, 18.10.2019 01:30 DragonWarrior203
Determine the ph of the following base solutions. (assume that all solutions are at 25°c and the ion-product constant of water, kw, is 1.01 ✕ 10−14.) (a) 1.39 ✕ 10−2 m naoh webassign will check your answer for the correct number of significant figures. (b) 0.0051 m al(oh)3 webassign will check your answer for the correct number of significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2


Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
Determine the ph of the following base solutions. (assume that all solutions are at 25°c and the ion...
Questions

Mathematics, 05.01.2020 17:31




Mathematics, 05.01.2020 17:31



Biology, 05.01.2020 17:31







Mathematics, 05.01.2020 17:31


Chemistry, 05.01.2020 17:31

Mathematics, 05.01.2020 17:31

Mathematics, 05.01.2020 17:31

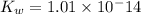
![K_w=[H^+][OH^-]](/tpl/images/0330/0905/bc68a.png)
![1.01\times 10^-{14}=[H^+][OH^-]](/tpl/images/0330/0905/ef4b3.png)
![-\log[1.01\times 10^-{14}]=(-\log [H^+])+(-\log [OH^-])](/tpl/images/0330/0905/fdeff.png)
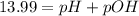
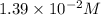 of NaOH.
of NaOH.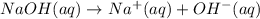
![[OH^-]=1\times [NaOH]=1\times 1.39\times 10^{-2} M=1.39\times 10^{-2} M](/tpl/images/0330/0905/52ef4.png)
![pOH=-\log[1.39\times 10^{-2} M]=1.86](/tpl/images/0330/0905/dac37.png)
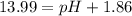
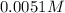 of NaOH.
of NaOH.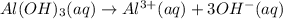
![[OH^-]=3\times [Al(OH)_3]=3\times 0.0051 M=0.0153 M](/tpl/images/0330/0905/db0a1.png)
![pOH=-\log[0.0153 M]=1.82](/tpl/images/0330/0905/47516.png)