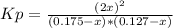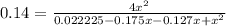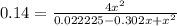Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatures. n2(g) + o2(g) ⇌ 2no(g)the equilibrium constant kp for the reaction is 0.14 at 1200 °c. if a container is charged with 0.175 atm of nitrogen and 0.127 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of oxygen? report your answer to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1


You know the right answer?
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatu...
Questions

Mathematics, 31.01.2020 08:51


English, 31.01.2020 08:51

Chemistry, 31.01.2020 08:51


History, 31.01.2020 08:51

Mathematics, 31.01.2020 08:51

Mathematics, 31.01.2020 08:52






Mathematics, 31.01.2020 08:52

English, 31.01.2020 08:52

Biology, 31.01.2020 08:52

Mathematics, 31.01.2020 08:52



Mathematics, 31.01.2020 08:52

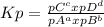 , where pX represents the partial pressure of X. So, for the reaction given, let's do a equilibrium table:
, where pX represents the partial pressure of X. So, for the reaction given, let's do a equilibrium table: