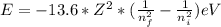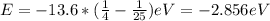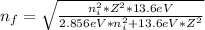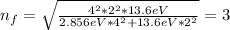Chemistry, 18.10.2019 18:30 gyexisromero10
When an excited electron in a hydrogen atom falls from =5 to =2, a photon of blue light is emitted. if an excited electron in an he+ ion falls from =4, which energy level must it fall to ) for blue light of a similar wavelength to be emitted?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
When an excited electron in a hydrogen atom falls from =5 to =2, a photon of blue light is emitted....
Questions


Mathematics, 14.07.2019 01:30

Mathematics, 14.07.2019 01:30

Health, 14.07.2019 01:30


History, 14.07.2019 01:30

Physics, 14.07.2019 01:30

Mathematics, 14.07.2019 01:30


Mathematics, 14.07.2019 01:30

Mathematics, 14.07.2019 01:30



Mathematics, 14.07.2019 01:30

Mathematics, 14.07.2019 01:30




Mathematics, 14.07.2019 01:30