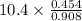Chemistry, 18.10.2019 19:10 Chrissyx4750
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in part a, and the temperature and volume were constant at values of 303 k and 2.00 l, respectively. if the pressure was 10.4 atm prior to the reaction, what would be the expected pressure after the reaction was completed?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in par...
Questions

Physics, 19.10.2019 11:30

English, 19.10.2019 11:30

Mathematics, 19.10.2019 11:30


Mathematics, 19.10.2019 11:30

Business, 19.10.2019 11:30


Mathematics, 19.10.2019 11:30


Mathematics, 19.10.2019 11:30

Spanish, 19.10.2019 11:30

Chemistry, 19.10.2019 11:30

Mathematics, 19.10.2019 11:30

History, 19.10.2019 11:30

Physics, 19.10.2019 11:30

Geography, 19.10.2019 11:30

Biology, 19.10.2019 11:30



Mathematics, 19.10.2019 11:30

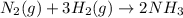
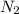 + moles of
+ moles of 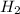 = 0.908 mol
= 0.908 mol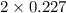 = 0.454 mol
= 0.454 mol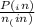 =
= 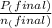
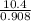 =
= 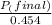
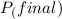 =
=