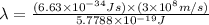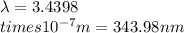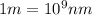Ultraviolet radiation and radiation of shorter wavelengths can damage biological molecules because they carry enough energy to break bonds within the molecules. a typical carbon–carbon bond requires 348 kj/mol to break. what is the longest wavelength of radiation with enough energy to break carbon–carbon bonds?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 05:00
Scientists discovered fossils in several layers of the earth you see here. they found fossils of algae, snails, and clams in layer d. given that information, where do you think they found fossil evidence of simple land plants and amphibians?
Answers: 1

Chemistry, 23.06.2019 11:30
What makes up a film badge? layers of photographic film covered in black paper. an electrolyte paste spread across a plastic sheet. a thin film of salts and other chemicals on clear acetate. a panel of photographic film exposed to the air. ✓
Answers: 2
You know the right answer?
Ultraviolet radiation and radiation of shorter wavelengths can damage biological molecules because t...
Questions

Mathematics, 30.08.2019 11:00



History, 30.08.2019 11:00

English, 30.08.2019 11:00

History, 30.08.2019 11:00

History, 30.08.2019 11:00

English, 30.08.2019 11:00



Spanish, 30.08.2019 11:00

Chemistry, 30.08.2019 11:00



Chemistry, 30.08.2019 11:00

History, 30.08.2019 11:00


Health, 30.08.2019 11:00

Biology, 30.08.2019 11:00

Mathematics, 30.08.2019 11:00

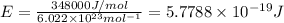
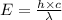
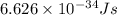
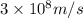
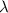 = wavelength of the radiation
= wavelength of the radiation