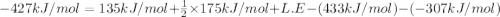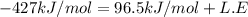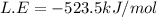Chemistry, 18.10.2019 19:20 ineedhelp2285
Consider an ionic compound, mx, composed of generic metal m and generic, gaseous halogen x. the enthalpy of formation of mxis δh∘f=−427kj/mol. the enthalpy of sublimation of mis δhsub=135kj/mol. the ionization energy of mis ie=433kj/mol. the electron affinity of xis δhea=−307kj/mol. (refer to the hint). the bond energy of x2is be=175kj/mol. determine the lattice energy of mx.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

You know the right answer?
Consider an ionic compound, mx, composed of generic metal m and generic, gaseous halogen x. the enth...
Questions

Mathematics, 02.10.2021 23:50



English, 02.10.2021 23:50



Business, 02.10.2021 23:50

Biology, 02.10.2021 23:50

Mathematics, 02.10.2021 23:50

Mathematics, 02.10.2021 23:50



Mathematics, 03.10.2021 01:00

Chemistry, 03.10.2021 01:00



Biology, 03.10.2021 01:00

History, 03.10.2021 01:00

Mathematics, 03.10.2021 01:00

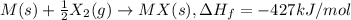 ....[1]
....[1]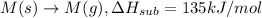 ....[2]
....[2]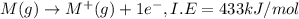 ....[3]
....[3]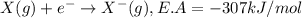 ....[4]
....[4]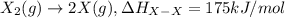 ....[5]
....[5] ....[6]
....[6]