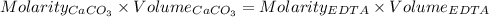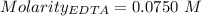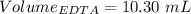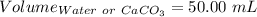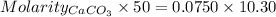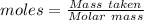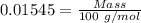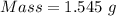A50.00 ml sample of groundwater is titrated with 0.0750 m edta . if 10.30 ml of edta is required to titrate the 50.00 ml sample, what is the hardness of the groundwater in molarity and in parts per million of caco3 by mass? assume that ca2+ accounts for all of the hardness in the groundwater.

Answers: 2


Another question on Chemistry




You know the right answer?
A50.00 ml sample of groundwater is titrated with 0.0750 m edta . if 10.30 ml of edta is required to...
Questions

Mathematics, 09.04.2020 23:55



English, 09.04.2020 23:55

Mathematics, 09.04.2020 23:55


History, 09.04.2020 23:55


Chemistry, 09.04.2020 23:55

Biology, 09.04.2020 23:55

History, 09.04.2020 23:55

Mathematics, 09.04.2020 23:55




Mathematics, 09.04.2020 23:55



History, 09.04.2020 23:55

Mathematics, 09.04.2020 23:55

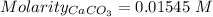
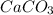 in water.
in water.