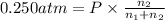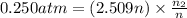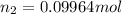Chemistry, 18.10.2019 19:20 arwasoliman363
The partial pressure of ch4 is 0.175 atm and that of o2 is 0.250 atm in a mixture of the two gases. what is the mole fraction of each gas in the mixture? if the mixture occupies a volume of 10.5 l at 35oc, calculate the total number of moles of gas in the mixture. calculate the number of grams of each gas in the mixture.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1


Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
The partial pressure of ch4 is 0.175 atm and that of o2 is 0.250 atm in a mixture of the two gases....
Questions

English, 05.07.2019 14:00


Biology, 05.07.2019 14:00

History, 05.07.2019 14:00


Mathematics, 05.07.2019 14:00

Mathematics, 05.07.2019 14:00

Geography, 05.07.2019 14:00

History, 05.07.2019 14:00

History, 05.07.2019 14:00

Computers and Technology, 05.07.2019 14:00

German, 05.07.2019 14:00

Biology, 05.07.2019 14:00

Mathematics, 05.07.2019 14:00

Mathematics, 05.07.2019 14:00

History, 05.07.2019 14:00

Social Studies, 05.07.2019 14:00


Geography, 05.07.2019 14:00

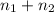
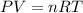
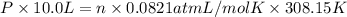
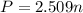
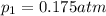
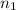
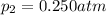
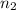
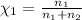
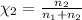
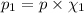 (Dalton's law)
(Dalton's law)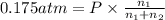
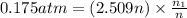
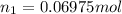
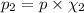 (Dalton's law)
(Dalton's law)