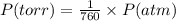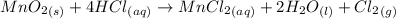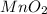Chemistry, 18.10.2019 21:00 lululoveee586
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, hcl(aq) , as described by the chemical equation mno2(s)+4hcl(aq)⟶mncl2(aq)+2h2o(l)+ cl2(g) how much mno2(s) should be added to excess hcl(aq) to obtain 255 ml cl2(g) at 25 °c and 725 torr ?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions


History, 13.11.2019 11:31

Social Studies, 13.11.2019 11:31

Biology, 13.11.2019 11:31

History, 13.11.2019 11:31

Mathematics, 13.11.2019 11:31

Chemistry, 13.11.2019 11:31

Mathematics, 13.11.2019 11:31





Mathematics, 13.11.2019 11:31

English, 13.11.2019 11:31



History, 13.11.2019 11:31