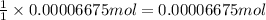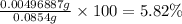Chemistry, 18.10.2019 22:00 hei40563273
In part 2 of the experiment, you will be analyzing a sample of household bleach. a 0.0854 g sample of household bleach is completely reacted with ki(s). the resulting solution is then titrated with 0.150 m nas2o3 solution. 0.890 ml of the solution is required to reach the colorless endpoint. what is the mass percent of naocl (mm = 74.44 g/mole) in the bleach? a. 30.1% b. 96.5% c. 2.23% d. 5.82%

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
In part 2 of the experiment, you will be analyzing a sample of household bleach. a 0.0854 g sample o...
Questions

Business, 30.11.2020 19:10





Mathematics, 30.11.2020 19:20

Business, 30.11.2020 19:20

Mathematics, 30.11.2020 19:20





English, 30.11.2020 19:20

Mathematics, 30.11.2020 19:20


Mathematics, 30.11.2020 19:20



Arts, 30.11.2020 19:20

Mathematics, 30.11.2020 19:20

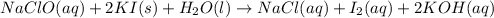 ..[1]
..[1]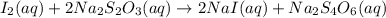 ..[2]
..[2]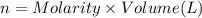
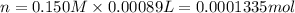
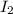 .
.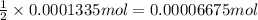 of
of