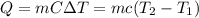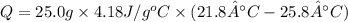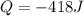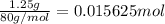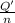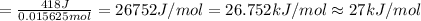Chemistry, 19.10.2019 00:00 tjacqueline9753
In order to measure the enthalpy change for this reaction, 1.25 g of nh4no3 is dissolved in enough water to make 25.0 ml of solution. the initial temperature is 25.8 degrees c and the final temperature (after the solid dissolves) is 21.9 degrees c. calculate the change in enthalpy for the reaction. (use 1.0g/ml as the density of the solution and 4.18 j/g . degrees c as the specific heat capacity.) express the answer to two significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which of the following can be used to measure electricity
Answers: 1

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
In order to measure the enthalpy change for this reaction, 1.25 g of nh4no3 is dissolved in enough w...
Questions

Mathematics, 12.11.2020 19:40

History, 12.11.2020 19:40

Mathematics, 12.11.2020 19:40


Mathematics, 12.11.2020 19:40

English, 12.11.2020 19:40




Biology, 12.11.2020 19:40


English, 12.11.2020 19:40



Biology, 12.11.2020 19:40

Biology, 12.11.2020 19:40


Business, 12.11.2020 19:40


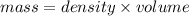
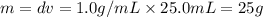
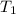 = 25.8°C
= 25.8°C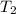 = 21.8°C
= 21.8°C