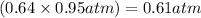Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
Amixture of he and ne at a total pressure of 0.95 atm is found to contain 0.32 mol of he and 0.56 mo...
Questions

Mathematics, 28.06.2019 10:20

English, 28.06.2019 10:20





Physics, 28.06.2019 10:20

History, 28.06.2019 10:20

Physics, 28.06.2019 10:20

Mathematics, 28.06.2019 10:20

Mathematics, 28.06.2019 10:20

Physics, 28.06.2019 10:20

History, 28.06.2019 10:20





Mathematics, 28.06.2019 10:20

Mathematics, 28.06.2019 10:20

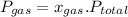
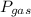 is partial pressure of a gas in mixture,
is partial pressure of a gas in mixture, 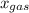 is mole fraction of a gas in mixture and
is mole fraction of a gas in mixture and 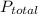 is total pressure of mixture.
is total pressure of mixture.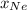 = (number of moles of Ne)/(Total number of moles in mixture)
= (number of moles of Ne)/(Total number of moles in mixture)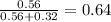
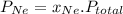 =
=