
Chemistry, 19.10.2019 00:30 hannahbannana98
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthalein as the indicator. in the reaction of oxalic acid with naoh, every 1 mole of oxalic acid is deprotonated by 2 moles of naoh. the molecular weight of oxalic acid is 90.03 g/mol and you use 0.60 g in 50 ml water for the titration. you use 15.20 ml of your naoh solution in order to reach the endpoint of the titration. what is the concentration of your naoh solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3


Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

You know the right answer?
You decide to titrate the solution of naoh using oxalic acid as a primary standard and phenolphthale...
Questions


Business, 21.12.2020 01:00

Mathematics, 21.12.2020 01:00

Mathematics, 21.12.2020 01:00

Mathematics, 21.12.2020 01:00





Computers and Technology, 21.12.2020 01:00





Mathematics, 21.12.2020 01:00


Engineering, 21.12.2020 01:00



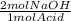 = 0.0133 mol NaOH
= 0.0133 mol NaOH

