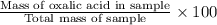Chemistry, 19.10.2019 00:30 reagriffis24
Oxalic acid, a diprotic acid having the formula h2c2o4, is used to clean the rust out of radiators in cars. a sample of an oxalic acid mixture was analyzed by titrating a 0.2816 g sample dissolved in water with 0.0461 m naoh. a volume of 11.49 ml of the base was required to completely neutralize the oxalic acid. what was the percentage by mass of oxalic acid in the sample?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Oxalic acid, a diprotic acid having the formula h2c2o4, is used to clean the rust out of radiators i...
Questions

Computers and Technology, 16.03.2022 18:40

Biology, 16.03.2022 18:40





SAT, 16.03.2022 18:50

Geography, 16.03.2022 18:50



English, 16.03.2022 18:50

Mathematics, 16.03.2022 18:50






Biology, 16.03.2022 18:50

History, 16.03.2022 18:50

Mathematics, 16.03.2022 18:50

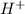 ions produced by
ions produced by 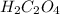 with total number of moles of
with total number of moles of 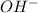 ions produced by NaOH.
ions produced by NaOH.
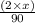 ........... (1) (We multiply by 2 because Oxalic Acid is a diprotic acid)
........... (1) (We multiply by 2 because Oxalic Acid is a diprotic acid)