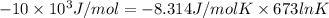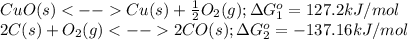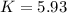Chemistry, 19.10.2019 02:30 patrickgonzalezjr13
Heating copper(ii) oxide at 400°c does not produce any appreciable amount of cu: cuo(s) ⇆ cu(s) + 1 2 o2(g) δg o = 127.2 kj/mol however, if this reaction is coupled to the conversion of graphite to carbon monoxide, it becomes spontaneous. write an equation for the coupled process and calculate the equilibrium constant for the coupled reaction. be sure to include the states of the chemical species.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Heating copper(ii) oxide at 400°c does not produce any appreciable amount of cu: cuo(s) ⇆ cu(s) + 1...
Questions

Biology, 09.07.2019 10:50




Chemistry, 09.07.2019 10:50


English, 09.07.2019 10:50

Mathematics, 09.07.2019 10:50

English, 09.07.2019 10:50




History, 09.07.2019 10:50



Mathematics, 09.07.2019 10:50

Geography, 09.07.2019 10:50

History, 09.07.2019 10:50


Advanced Placement (AP), 09.07.2019 10:50

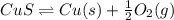
 is positive. Therefore, reaction is non-spontaneous.
is positive. Therefore, reaction is non-spontaneous.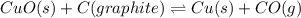

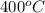 = (400 + 273) K = 673 K
= (400 + 273) K = 673 K