Chemistry, 19.10.2019 02:30 mechelllcross
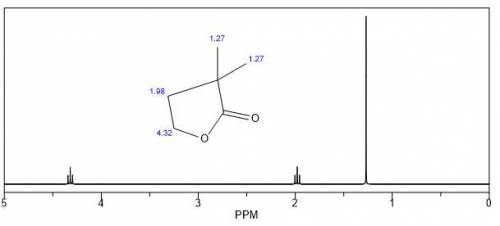
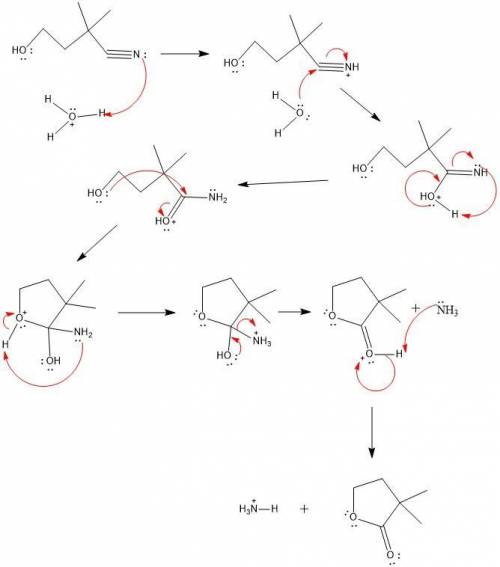
Acid catalyzed hydrolysis of hoch2ch2c(ch3)2cn forms compound a (c6h10o2). a shows a strong peak in its ir spectrum at 1770 cm−1 and the following signals in its 1 h nmr spectrum: 1.27 (singlet, 6 h), 2.12 (triplet, 2 h), and 4.26 (triplet, 2 h) ppm. draw the structure for a and select the correct answers to complete a mechanism that accounts for its formation.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
You know the right answer?
Acid catalyzed hydrolysis of hoch2ch2c(ch3)2cn forms compound a (c6h10o2). a shows a strong peak in...
Questions

Mathematics, 16.06.2021 01:00











Chemistry, 16.06.2021 01:00





Computers and Technology, 16.06.2021 01:00

Mathematics, 16.06.2021 01:00

Mathematics, 16.06.2021 01:00

Mathematics, 16.06.2021 01:00