
This question has multiple parts.
a. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with 85.00 g water at an initial temperature 25.00oc. after dissolution of the salt, the final temperature of the calorimeter contents was 23.34oc.
assuming the solution has a heat capacity of 4.18 j/g∙oc, and assuming no heat loss to the calorimeter, calculate the enthalpy of solution (∆hsoln) for the dissolution of nh4no3 in units of kj/mol.
∆ = kj/mol
b. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with 85.00 g water at an initial temperature 25.00oc. after dissolution of the salt, the final temperature of the calorimeter contents was 23.34oc.
if the enthalpy of hydration for nh4no3 is -630. kj/mol, calculate the lattice energy of nh4no3.
lattice energy = kj/mol

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 22:00
For a family dinner, jose’s mom baked a loaf of bread. during the meal, his grandmother commented, “this bread is so dense! ” what do you think his grandmother meant by the word dense? you have some ideas regarding density already, but this experiment will enrich your understanding of the relationship between mass, volume and the density of objects. when making measurements in this experiment we will be using si units, the international system of units. the purpose of using si units is to have the ability to communicate and compare data results with other experiments without having to make conversions. measurement symbol si unit length m meter mass kg kilogram volume l liters density kg/l kilograms per liter any calculations that are performed during this experiment will ultimately be reported in scientific notation. recall that scientific notation is extremely important when we have data that is extremely small or large. scientists rely on a standard form of communication to quickly make hypotheses and judgements based on data. what is the density of block a? a0kg/l what is the density of block b? a1kg/l what is the density of block c? a2kg/l what is the density of block d? a3kg/l what is the density of block e? a4kg/l which block is the densest? a5
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
This question has multiple parts.
a. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with...
a. in a coffee-cup calorimeter, 1.81 g nh4no3 was mixed with...
Questions

English, 28.12.2019 08:31



Physics, 28.12.2019 08:31



History, 28.12.2019 08:31


Mathematics, 28.12.2019 08:31


Chemistry, 28.12.2019 08:31




Chemistry, 28.12.2019 08:31


English, 28.12.2019 08:31

Biology, 28.12.2019 08:31


Mathematics, 28.12.2019 08:31

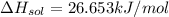

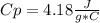
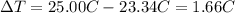
![m=m_{water} + m_{salt]=1.81g +85g= 86.81g](/tpl/images/0333/2995/cfc86.png)
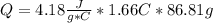
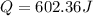
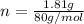
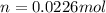
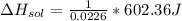
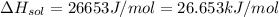
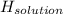 = 26.20 kJ/mol
= 26.20 kJ/mol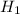 ) = - 656.20 kJ/mol
) = - 656.20 kJ/mol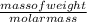
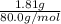

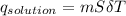
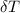 = 25.00°C - 23.34°C
= 25.00°C - 23.34°C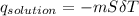 (since heat is lost by the water to the compound)
(since heat is lost by the water to the compound)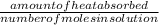
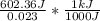
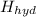 + Δ
+ Δ

