
Chemistry, 19.10.2019 04:20 viktoria1198zz
If the percent yield for the following reaction is 80.37%, and 63.21 g of no2 are used in the reaction mixture, how many grams of nitric acid, hno3(aq), are produced in the experiment? 3 no2(g) + h2o(l) → 2 hno3(aq) + no(g) molar mass no2 = 46.01 g/mol and hno3 = 63.01 g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3


Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
If the percent yield for the following reaction is 80.37%, and 63.21 g of no2 are used in the reacti...
Questions

Chemistry, 27.07.2019 03:00

English, 27.07.2019 03:00

History, 27.07.2019 03:00

Business, 27.07.2019 03:00


Mathematics, 27.07.2019 03:00


Mathematics, 27.07.2019 03:00

Mathematics, 27.07.2019 03:00

History, 27.07.2019 03:00


History, 27.07.2019 03:00

Mathematics, 27.07.2019 03:00


English, 27.07.2019 03:00

History, 27.07.2019 03:00

Geography, 27.07.2019 03:00

Business, 27.07.2019 03:00

History, 27.07.2019 03:00

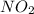 is 46.01 g/mol and mass of
is 46.01 g/mol and mass of 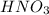 is 63.01 g/mol.
is 63.01 g/mol.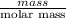
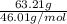
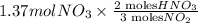
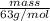
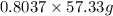
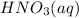 , are produced in the experiment.
, are produced in the experiment.

