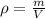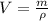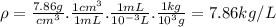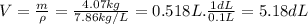Chemistry, 19.10.2019 04:20 elawnnalewis7486
Iron has a density of 7.86 g/cm3. calculate the volume (in dl) of a piece of iron having a mass of 4.07 kg . note that the density is provided in different units of volume and mass than the desired units of volume (dl) and the given units of mass (kg). you will need to express the density in kg/dl (1 cm3 = 1 ml) before calculating the volume for the piece of iron.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Iron has a density of 7.86 g/cm3. calculate the volume (in dl) of a piece of iron having a mass of 4...
Questions

Mathematics, 10.07.2019 20:30




English, 10.07.2019 20:30







Mathematics, 10.07.2019 20:30



History, 10.07.2019 20:30





Physics, 10.07.2019 20:30