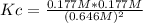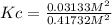Chemistry, 21.10.2019 18:10 lejeanjamespete1
Consider the reaction: 2hi(g) ⇄ h2(g) + i2(g). it is found that, when equilibrium is reached at a certain temperature, hi is 35.4 percent dissociated. calculate the equilibrium constant kc for the reaction at this temperature?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
Consider the reaction: 2hi(g) ⇄ h2(g) + i2(g). it is found that, when equilibrium is reached at a c...
Questions




Computers and Technology, 11.03.2021 03:40

Mathematics, 11.03.2021 03:40

Mathematics, 11.03.2021 03:40

Mathematics, 11.03.2021 03:40


French, 11.03.2021 03:40

Mathematics, 11.03.2021 03:40


Biology, 11.03.2021 03:40

Mathematics, 11.03.2021 03:40






Mathematics, 11.03.2021 03:40

![Kc = \frac{[H2]*[I2]}{[HI]^2}](/tpl/images/0338/7477/a79bd.png)