Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
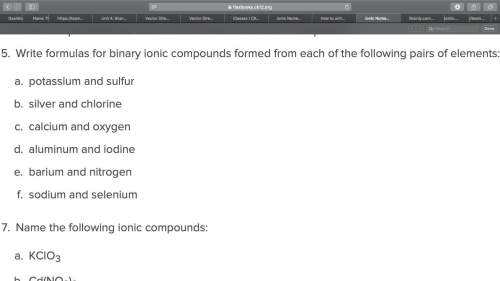
Write formulas for binary ionic compounds formed from each of the following pairs of elements
...
...
Questions

Mathematics, 14.12.2020 23:10


Engineering, 14.12.2020 23:10

Chemistry, 14.12.2020 23:10


Mathematics, 14.12.2020 23:10



Mathematics, 14.12.2020 23:10

Computers and Technology, 14.12.2020 23:10


Mathematics, 14.12.2020 23:10



Mathematics, 14.12.2020 23:10

Medicine, 14.12.2020 23:10



Mathematics, 14.12.2020 23:10

Chemistry, 14.12.2020 23:10