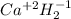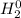Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2


Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Classify each of the following binary compounds by the oxidation number of hydrogen. match each item...
Questions


Mathematics, 11.10.2020 14:01




Physics, 11.10.2020 14:01

Chemistry, 11.10.2020 14:01

English, 11.10.2020 14:01

Advanced Placement (AP), 11.10.2020 14:01



Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01



World Languages, 11.10.2020 14:01



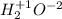 ,
, 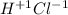 ,
, 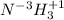 ,
, 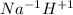 ,
,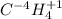
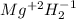 ,
,