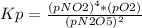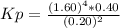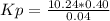Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 10:50
Which compound should undergo substitution of the bromine by phenolate anion? draw the structure of the organic product?
Answers: 1
You know the right answer?
1.00 atm n2o5 is placed in an evacuated vessel and reacts according to the equation 2 n2o5(g) ⇄ 4 no...
Questions








Arts, 13.03.2020 22:23

Mathematics, 13.03.2020 22:24





Computers and Technology, 13.03.2020 22:24




Mathematics, 13.03.2020 22:24

English, 13.03.2020 22:24