
Chemistry, 21.10.2019 23:30 rachelsweeney10
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then adds iron powder to the conical flask at once. then, she stirs it briskly to initiate and facilitate the reaction between copper sulfate and iron powder. while stirring continuously, vanessa observes that the blue color of copper sulfate solution is fading substantially. moreover, she also observes the deposition of a reddish-brown mass at the bottom of the flask.
finally, a stage is reached where the solution becomes pale green in color. on further stirring of the solution, there is no change in the intensity of color. a distinct reddish-brown layer is deposited in the conical flask. however, on weighing the reaction mixture, she finds that the mass of the reaction mixture remains unchanged at 215.4 g. the reddish-brown layer is then separated from the pale green solution and allowed to dry in an oven. the moisture is completely evaporated, and the dried layer is weighed. its weight is found to be 63.5 g.
what is the mass of required iron powder and the formed iron sulfate in the chemical reaction?
a
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
b
the mass of iron powder required to complete the reaction was 63.5 g, while the mass of iron sulfate formed in the reaction was 151.9 g.
c
the mass of iron powder required to complete the reaction was 159.6 g, while the mass of iron sulfate formed in the reaction was 63.5 g.
d
the mass of iron powder required to complete the reaction was 55.8 g, while the mass of iron sulfate formed in the reaction was 159.6 g.
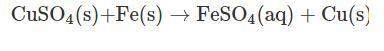

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 15:40
The table below shows the freezing points of four substances. substance freezing point (°c) benzene 5.5 water 0 butane –138 nitrogen –210 the substances are placed in separate containers at room temperature, and each container is gradually cooled. which of these substances will solidify before the temperature reaches 0°c? benzene water butane nitrogen
Answers: 2
You know the right answer?
Vanessa weighs 159.6 gms of copper sulfate solution and transfers it into a conical flask. she then...
Questions

Spanish, 21.02.2021 20:00



Mathematics, 21.02.2021 20:10

Mathematics, 21.02.2021 20:10


Geography, 21.02.2021 20:10




History, 21.02.2021 20:10

Mathematics, 21.02.2021 20:10

Health, 21.02.2021 20:10





Computers and Technology, 21.02.2021 20:10

Mathematics, 21.02.2021 20:10




