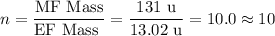Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
The empirical formula of a compound is ch. at 200 degrees celsius, 0.242 of this compound occupies 9...
Questions


Mathematics, 06.02.2021 03:50

Mathematics, 06.02.2021 03:50

Physics, 06.02.2021 03:50

Mathematics, 06.02.2021 03:50

Mathematics, 06.02.2021 03:50


Biology, 06.02.2021 03:50

Chemistry, 06.02.2021 03:50

English, 06.02.2021 03:50





Mathematics, 06.02.2021 03:50

History, 06.02.2021 03:50

Social Studies, 06.02.2021 03:50

English, 06.02.2021 03:50