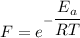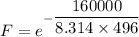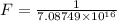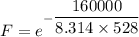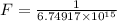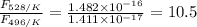The activation energy for the isomerization of methyl isonitrile is 160 kj/mol. part acalculate the fraction of methyl isonitrile molecules that have an energy of 160.0 kj or greater at 496 k .f1=part bcalculate this fraction for a temperature of 528 k .f2=part cwhat is the ratio of the fraction at 528 k to that at 496 k ? f2/f1=

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
The activation energy for the isomerization of methyl isonitrile is 160 kj/mol. part acalculate the...
Questions

Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

History, 23.04.2021 20:20





Mathematics, 23.04.2021 20:20

English, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

History, 23.04.2021 20:20



Social Studies, 23.04.2021 20:20

Chemistry, 23.04.2021 20:20


Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

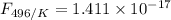
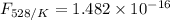
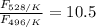
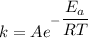
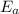 is the activation energy
is the activation energy
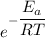 is the fraction of molecules which have value equal to or grater than activation energy.
is the fraction of molecules which have value equal to or grater than activation energy.