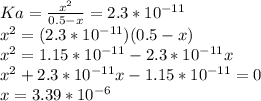Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

You know the right answer?
Calculate the ph of a 0.50 m hio. the ka of hypoiodic acid, hio, is 2.3x10–11.0.305.325.479.474.80...
Questions


Mathematics, 09.12.2019 21:31

English, 09.12.2019 21:31




Mathematics, 09.12.2019 21:31

Mathematics, 09.12.2019 21:31



Biology, 09.12.2019 21:31

Social Studies, 09.12.2019 21:31








![\frac{[H+][IO-]}{[HIO]}](/tpl/images/0340/0266/e2430.png) = 2.3 * 10⁻¹¹
= 2.3 * 10⁻¹¹