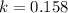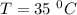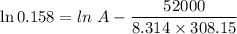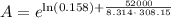Chemistry, 22.10.2019 04:00 Kingdcn6261
For a reaction with an activation energy of 52.0 kilojoules per mole and at a temperature of 35°c find the pre-exponential factor if the rate constant is 0.158 a) 0.161 в) 1.03 x 10% c) 6.42 x 1076 10 d) 2.42 x 10% e) 6.71 x 106

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
For a reaction with an activation energy of 52.0 kilojoules per mole and at a temperature of 35°c fi...
Questions

Computers and Technology, 25.03.2020 05:53


Mathematics, 25.03.2020 05:53




Social Studies, 25.03.2020 05:54

Mathematics, 25.03.2020 05:54



English, 25.03.2020 05:54









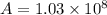
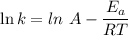
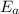 is the activation energy
is the activation energy