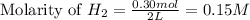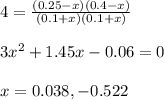The water-gas shift reaction plays a central role in the chemical methods for obtaining cleaner fuels from coal: co(g) + h2o(g) ⇌ co2(g) + h2(g) a study was made in which equilibrium was reached with [co] = [h2o] = [h2] = 0.10 m and [co2] = 0.40 m. after 0.30 mol of h2 is added to the 2.0−l container and equilibrium is reestablished, what are the new concentrations of all the components?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1


Chemistry, 23.06.2019 13:30
The zinc within a copper-plated penny dissolves in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can reach the zinc). the reaction between the acid and the zinc 2h+(aq)+zn(s)→h2(g)+zn2+(aq) . when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 °c is 0.947 l at a total pressure of 743 mmhg . (vapor pressure of water is 23.78 mmhg at 25 °c .) what mass of hydrogen gas is collected? answer in appropriate significant figures
Answers: 3
You know the right answer?
The water-gas shift reaction plays a central role in the chemical methods for obtaining cleaner fuel...
Questions

Biology, 04.02.2020 06:06

Mathematics, 04.02.2020 06:06

Mathematics, 04.02.2020 06:06


Mathematics, 04.02.2020 06:06

English, 04.02.2020 06:06



History, 04.02.2020 06:06

Mathematics, 04.02.2020 06:06

History, 04.02.2020 06:06

History, 04.02.2020 06:06

English, 04.02.2020 06:06


History, 04.02.2020 06:06



Biology, 04.02.2020 06:06


Mathematics, 04.02.2020 06:06

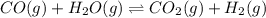
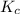 for above reaction follows:
for above reaction follows:![K_c=\frac{[CO_2][H_2]}{[CO][H_2O]}](/tpl/images/0341/1452/fbbde.png) ........(1)
........(1)![[CO]_{eq}=[H_2O]_{eq}=[H_2]_{eq}=0.10M](/tpl/images/0341/1452/8e2c6.png)
![[CO_2]_{eq}=0.40M](/tpl/images/0341/1452/b62fe.png)