
Chemistry, 22.10.2019 20:00 loistaylor1819
Achemist dissolves silver nitrate (agno₃) in water. he also dissolves ammonium chloride (nh₄cl) in water. he mixes the two solutions. a precipitate of silver chloride (agcl) forms. which chemical equation correctly represents this reaction?
a. agno₃(s) + nh₄cl(s)  agcl(s) + nh₄no₃(s)
agcl(s) + nh₄no₃(s)
b. agno₃(aq) + nh₄cl(aq)  agcl(aq) + nh₄no₃(aq)
agcl(aq) + nh₄no₃(aq)
c. agno₃(aq) + nh₄cl(aq)  agcl(s) + nh₄no₃(aq)
agcl(s) + nh₄no₃(aq)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 08:30
Which can be observed only in a microscopic view? a) structure of a muscle cell b) shape of a soybean plant c) foam insulation d) x-ray of a knee joint
Answers: 2
You know the right answer?
Achemist dissolves silver nitrate (agno₃) in water. he also dissolves ammonium chloride (nh₄cl) in w...
Questions

History, 22.02.2021 21:50



Mathematics, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50


English, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50




Health, 22.02.2021 21:50



Biology, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50


English, 22.02.2021 21:50


Mathematics, 22.02.2021 21:50

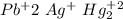
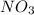 and ammonium salts
and ammonium salts 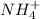 are soluble.
are soluble.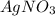 ,
,  and
and 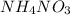 would be soluble, so we have to use the aqueosus state (aq).
would be soluble, so we have to use the aqueosus state (aq).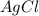 then would have a solid state (s).
then would have a solid state (s).

